0
UT100CS Handheld Capnography Monitor - SPO2 Sidestream ETCO2 PR RESP
AU$1,539.00
Sold out
Sold out
Product Details
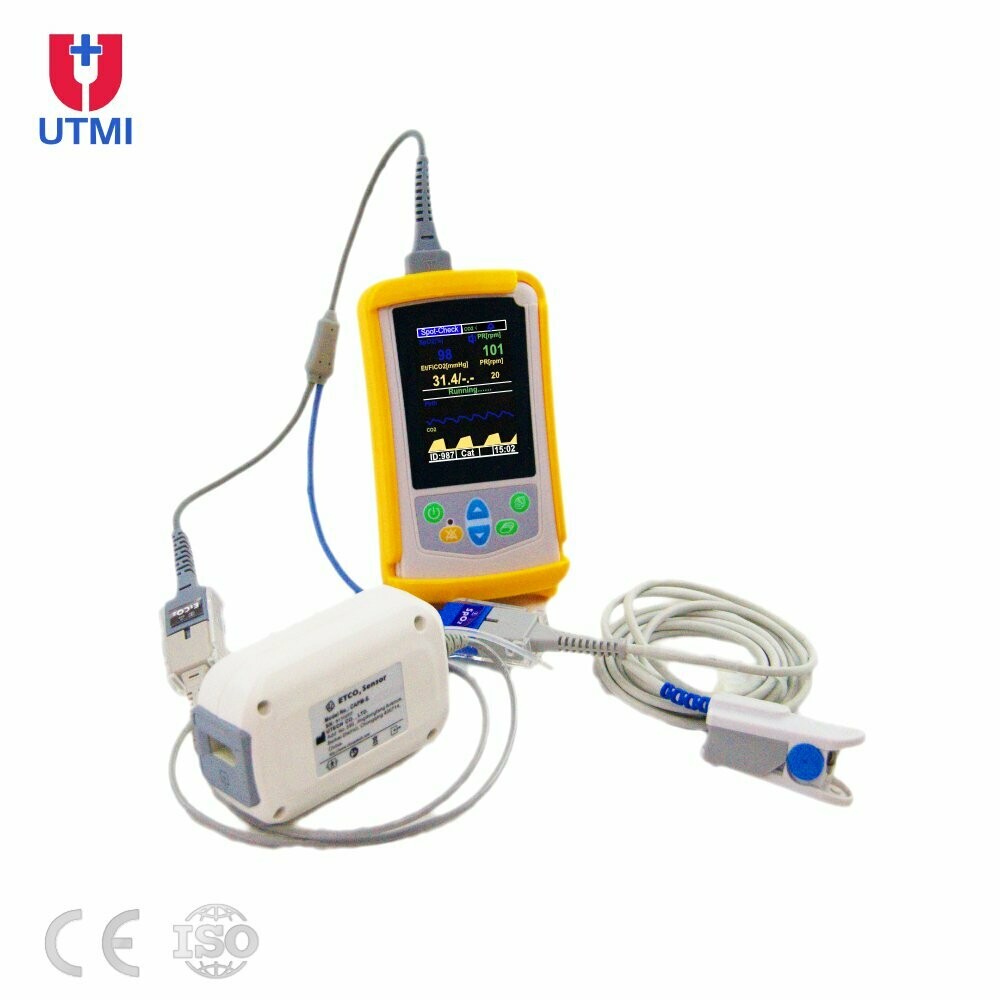
UT100C Handheld Capnograph ETCO2 Monitor are commonly used devices in clinics, hospitals and for first aid, emergency diagnostic and monitoring situations. They measure the patient's blood oxygen saturation (SpO2%) level and Pulse Rate, ETCO2 and RESP, vital indicators of many conditions/ health status
Main Features:
- mall and light, ergonomically designed to fit comfortably in the palm of your hand
- 2.8 inch large-sized colourful screen, bright, easy to read LCD displays indicate SpO2, ETCO2 and pulse rate measurements, plethysmogram and trend table
- Screen rotation provides upright display for vertical or horizontal monitoring position
- Perfusion index indicates arterial pulse signal strength
- Two measuring modes:
- Spot-check mode: measure data intermittently
- Monitor mode: measure and store data continuously
- Quick, reliable and accurate readings on SpO2, ETCO2 and Pulse rate
- 120 hours record storage
- Visual and audible alarm
- Adjustable volume (including silence) "beep" sounds with each pulse beat
- Patient information management. Patient's information such as ID, Sex, Type can be edited
- USB interface. Data can be transferred to PC through a data line for storage, review and analysis
- Low power consumption, long operation time (more than 20 hours)
- Turn off automatically to save power
- Uses 4 standard AA alkaline or Ni-MH Batteries
- Low battery Alarm. Low battery icon flashes when about 15 minutes of battery use remains.
- 4 display modes:
- Big Display Mode
- Waveform Display Mode
- Trend-table Display Mode
- Horizontal Display Mode
- 1Multi-languages: English, French, Spanish, Germany, Chinese
- AC power jack. An optional to connect to AC power supply
| Technical Specification | |||||
| Enclosure | |||||
| Weight | 258grams (9.1 ounces) with batteries | ||||
| Dimensions | 13.5cm × 7.5cm × 2.8cm | ||||
| Alarms | |||||
| Categories | Patient status and system status | ||||
| Notification | Audible and visual | ||||
| Setting | Full storage, low battery and sensor off | ||||
| Range | Pulse rate (30-350bpm), SpO2 (0-100%) | ||||
| Alarm volume level | 0-85dB | ||||
| Range, Accuracy and Resolution | |||||
| Range Type | Range Values | ||||
| Measurement Ranges | |||||
| SpO2 | 0-100% | ||||
| Pulse rate | 30-350bpm | ||||
| RESP | 0-150bpm | ||||
| ETCO2 | 0-20% (0-150mmHg) | ||||
| Measurement Accuracy | |||||
| SpO2 | 70-100% (±2%), <70% undefined | ||||
| Pulse rate | ±2bpm/±2% take maximum | ||||
| ETCO2 | ±2 mm Hg @ < 5.0% CO2 (at BTPS) < 10% of reading @ > 5.0% CO2(at BTPS) |
||||
| RESP | ±1rpm | ||||
| Resolution | |||||
| SpO2 | 1% | ||||
| Pulse rate | 1bpm | ||||
| ETCO2 | 0.1mmHg | ||||
| RESP | 1rpm | ||||
| Electrical | |||||
| AC power requirement | 100-240VA, 47-63Hz | ||||
| Power comsumption | 20VA | ||||
| Batteries | |||||
| Types | Alkaline/Ni-Mh Batteries, IEC: LR6 | ||||
| Operating time | ≥20hours without ETCO2;≤10hours with ETCO2 | ||||
| Charge time | ≤ 6hours | ||||
| Environment | |||||
| Operating tempearture | 0-45℃ | ||||
| Storage temperature | -20-60℃ | ||||
| Operating humidity | 30-95% | ||||
| Operating altitude | -500-5000m | ||||
| Trends | |||||
| Types | Tabular | ||||
| Memory | Save total 120 hours data events. Save data and time, alarm conditions, pulse rate and SpO2 measurements | ||||
| Standards and Certification | |||||
| IEC 62366 | Medical devices-Application of usability engineering to medical devices | ||||
| IEC 62304 | Medical device-software life cycle processes | ||||
| MDD 93/42/EEC | European Medical Device Directive | ||||
| ISO 13485 | Medical equipment-Quality management system | ||||
| EN ISO 14971 | Medical devices-Risk management | ||||
| EN 60601-1 | Medical electrical equipment | ||||
| EN 60601-2 | Medical electrical equipment | ||||
| EN 60601-8 | Medical electrical equipment | ||||
| EN 980 | Symbols for use in labeling of medical devices | ||||
| EN 1041 | Information supplied by the manufacture of medical devices | ||||
| ISO 80601-2-61 | Medical electrical equipment-Particular requirements for the basic safety and essential performance of pulse oximeter equipment for medical use | ||||
| EN ISO 10993-1 | Biological evaluation of medical devices | ||||
| EN ISO 10993-5 | Biological evaluation of medical devices | ||||
| EN ISO 10993-10 | Biological evaluation of medical devices | ||||
| ISO 14155-1 | Clinical investigation of medical devices | ||||
| CE | CONFORMITE EUROPEENNE | ||||
| SFDA | State Food and Drug Administration | ||||
Standard Configuration
- Handheld UT100C main unit
- Adult Finger Clip SpO2 sensor
- Sidestream ETCO2 Sensor
- Nasal Sampling Line
- Gas Sampling Line
- Gas Dryer Line
- T-fitting Tube
- Sidestream Adapter
- Adapter Cable
- Hanging Strap
- Protective Cover
- User Manual
Optional Accessories:
- Carrying Bag
- ODMS (Oximetry Data Management System)
- Ni-NH Rechargeable Cells/ Batteries
- Alkaline Cells/ Batteries
Warranty:
Main unit 2 Years
Accessories 6 Months
UT100CS Handheld Capnography Monitor - SPO2 Sidestream ETCO2 PR RESP
Display prices in:
AUD